Process Validation Protocol Template
This process validation protocol is applicable to carry out process validation of name of the product for first three consecutive commercial batches in view of the requirements of name of market at formulation plant of pharmaceutical company.
Process validation protocol template. Quality safety and efficacy are designed or built into the product. Protocol authors should never be allowed to commence construction of validation template protocols without being made aware of the company constraints definitions scopes methodology and individual responsibilities as authorized by the company. Misconceptions about process validation validation protocols and the rule of three in addition to revisiting important long standing principles of process validation by linking them to regulatory requirements the 2011 guidance dispels common misconceptions about process validation. Process validation protocol pharmaceutical template pdf ppt xls this is to assure drug quality.
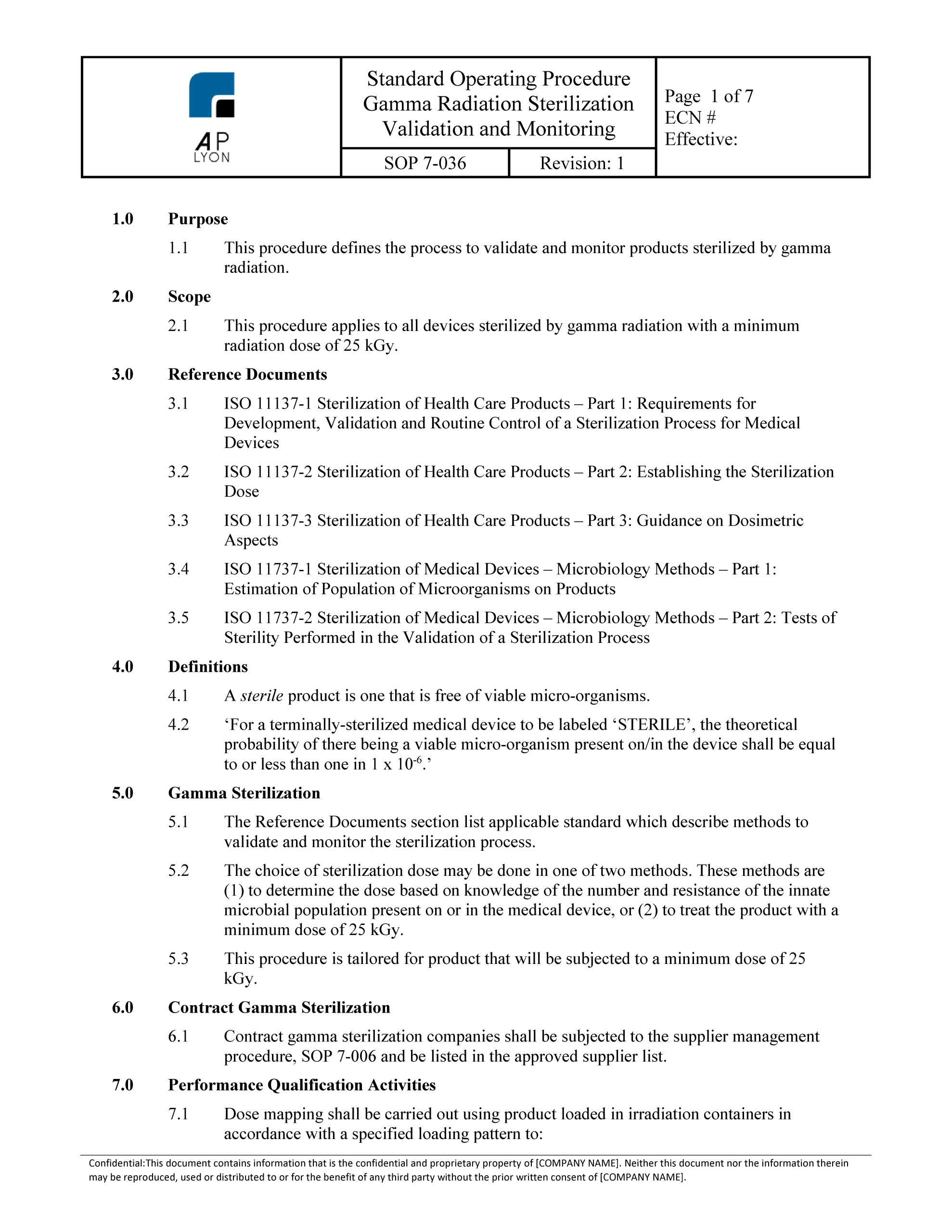
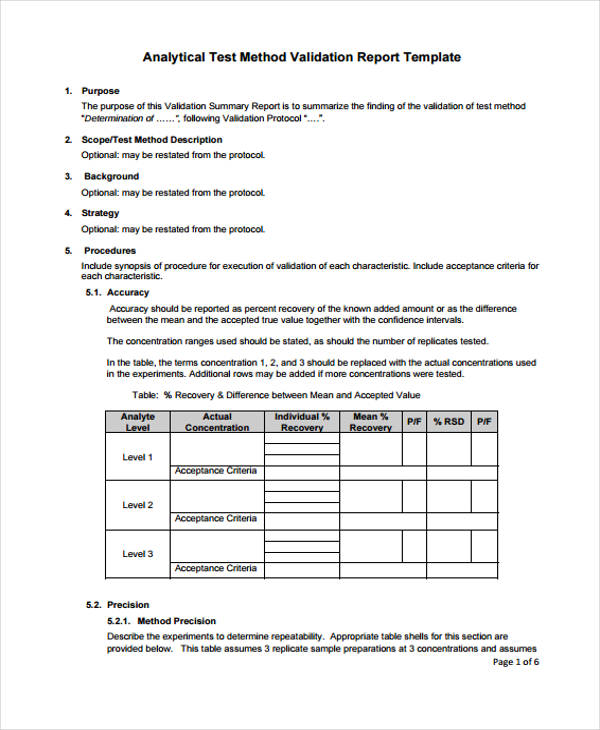
Technical services approves the validation protocol and report and reviews the executed test scripts and any validation deviations. Template for process validation protocol free download as word doc doc docx pdf file pdf text file txt or read online for free. Study this protocol was generated and approved to validate a high performance liquid chromatographic hplc stability indicating method for the analysis of compound a and its impurities related a and related b in your product 5 and 10 mg tablets. Process validation template types format pdf.
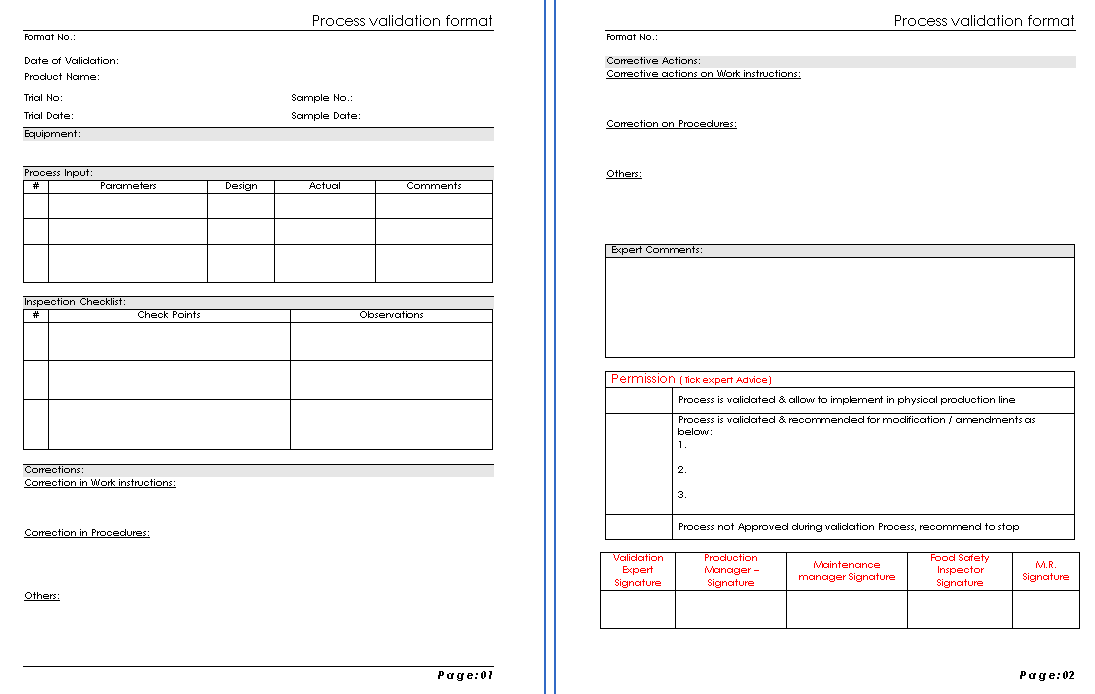
Sop page 14 of 24 10. Process validation deviations deviations from the signed and approved methodology procedure or expected versus actual results will be recorded on the deviation log and summary form in appendix 7 and categorized as critical and non critical. Template to carry for validation. It is a example for the validation protocol.
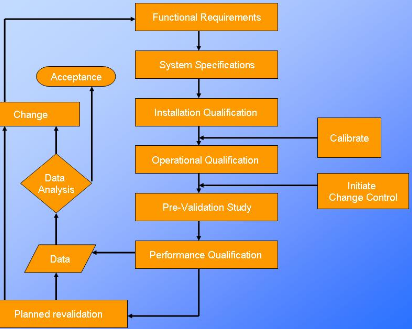
Validation protocol report format types pdf ppt. Validation scopes boundaries and responsibilities must be set out in the validation plan vp. Process validation protocol template or format for the products manufactured in the pharmaceutical product manufacturing facility. Process validation principle incorporates the understanding that the following conditions exist.
Template for an example methods validation protocol 171 i. This individual is responsible for ensuring that the validation study is practical follows sound validation principles and methodology and is in accordance with requirements and all applicable policies.