Eu Mdr Technical Documentation Template
Medical devices notified body bsi bsi uk bsi nl and medical device manufacturers both have an interest in.
Eu mdr technical documentation template. It was just good practice. The mdr is signi icantly more prescriptive about the required content of technical documentation technical filedesign dossier. First you need to know that the eu mdr 2017745 is providing a clear view of what should contain a technical file when the mdd 9342ec was not so structured. 2017 and was published in the of icial journal of the european union on 5th may 2017.
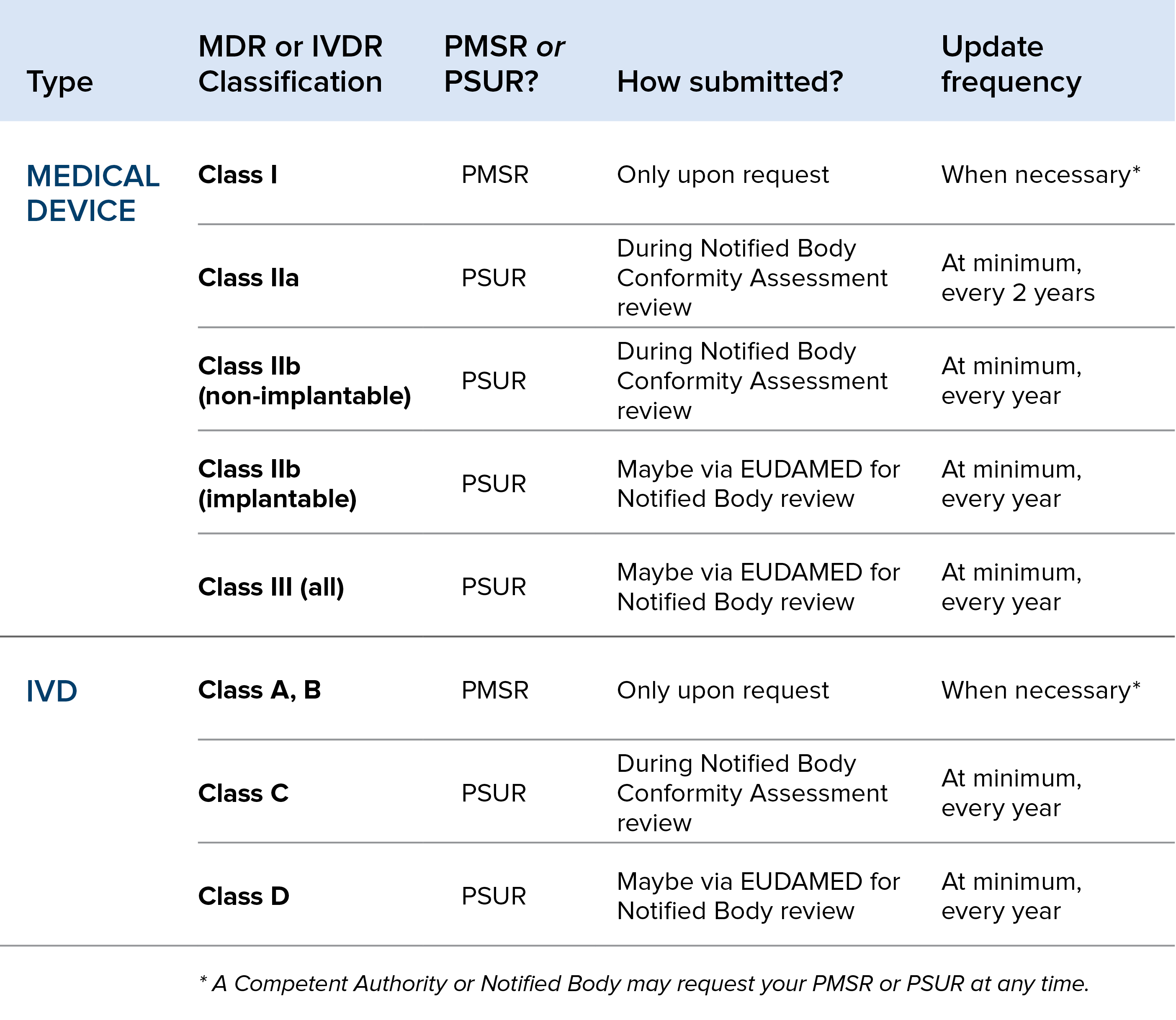
This document provides a strategic overview of the impact of medical device regulation mdr impact on our sector and is addressed to all parties involved in implementing the mdr. It outlines cocirs views on four key strategic areas set out in the. The mdds design dossier has been dropped from the eu mdr. Plus annex iii of the eu mdr requires more than fifteen additional elements in the technical documentation on post market surveillance.
A lot of information will have been produced in the previous steps. The technical documentation submission guidance is aligned to the requirements of eu 2017745 medical devices regulation mdr described in detail in annex ii and iii of eu 2017745. This new regulation whose full name is. This eu mdr technical documentation template will provide you all the necessary information that you need to gather.
A it will conform to the eus technical requirements b it will achieve its intended medical purpose and be safe to use and c that proper processes were followed to generate the information which supports the. Compile your eu technical file or design dossier with internal peer review. That information needs to be organised and presented in a way that demonstrates if the device is approved. Article 10 of the eu mdr makes the creation and maintenance of both parts of the technical documentation an obligation of all.
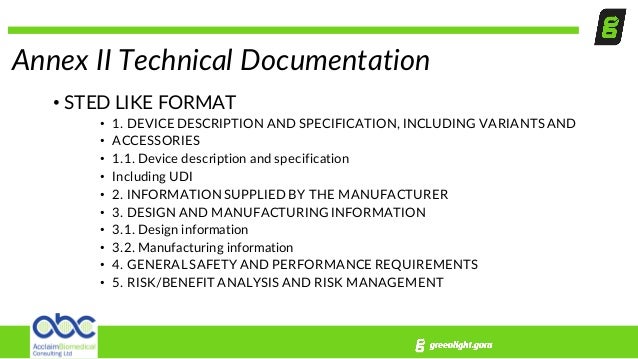
Fortunately imrdf or ghtf created a template called sted summary technical documentation medical device to help organize all the information but this was not mandatory per legislation. Structure of technical documentation medical devices 001122018 id. Completely review all existing documentation in support of meeting the applicable essential requirements of the directives. Prac cal implica ons for manufacturers.
Evaluate and identify gaps or deficiencies in your documentation. This technical documentation template conforms to eu mdr 2017745 annex ii and annex iii requirements. 2379 page 1 of 4 the following structure is based on regulation eu 2017745 mdr but is also suitable for technical documentation according to di.