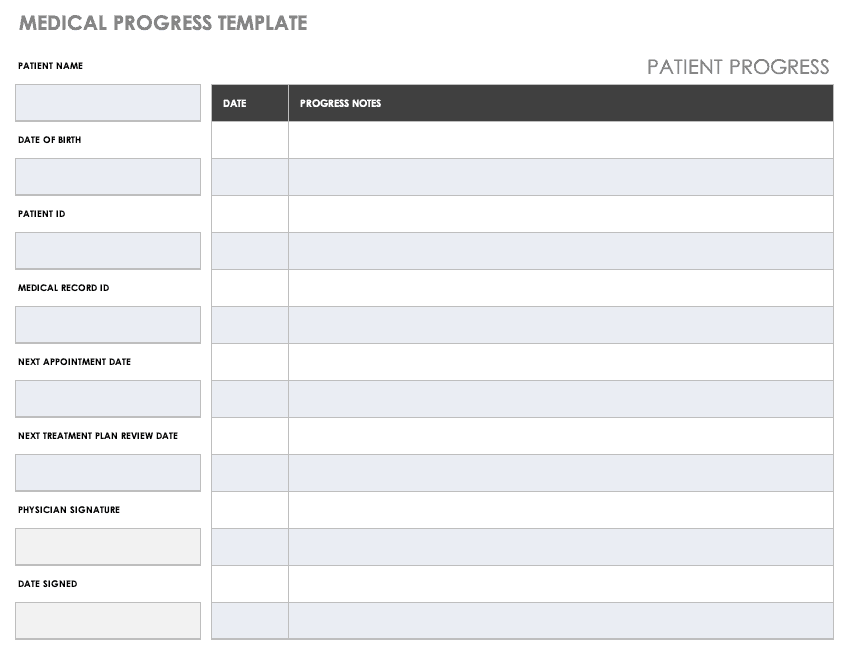
Medical Protocol Template

For device validation to perform in this environment the production testing of fda medical device templates must have both compliant procedures and an excellent technical strategy.
Medical protocol template. Independent expert to monitor. Sample protocol template. The first type of trials are phase 2 and 3 clinical trial protocols that require a food and drug administration fda investigational new drug ind or investigational device exemption ide application. American college of radiology imaging network acrin acrin 4701record procurement unit.
Hipaa compliant release of patient information pursuant to 45 cfr 164508. Institutional data and safety monitoring board. Definitions of adverse events. Independent data and safety monitoring board.
At the same time the fda medical device templates business has become highly regulated. This protocol template aims to facilitate the development of two types of clinical trials involving human participants. Protocol template with guidelines. This template should only be used for for studies limited to 1 the use of existing data or specimens 2 where the only study procedure is a retrospective chart review or use of existing biological samples and 3 where the analysis plan is limited to purely descriptive summary statistics.
Protocol template download version.