Mdr Gap Analysis Template
Global impact analysis template to provide a plan country by country.
Mdr gap analysis template. Types of new test data necessary for mdr compliance. One thing that seems to be constant in the global medical device industry is change. The use of the gap assessment templates will be explained if applicable in such sessions. Mdr readiness review revision 1 july 2017 page 6 of 9 safety and performance.
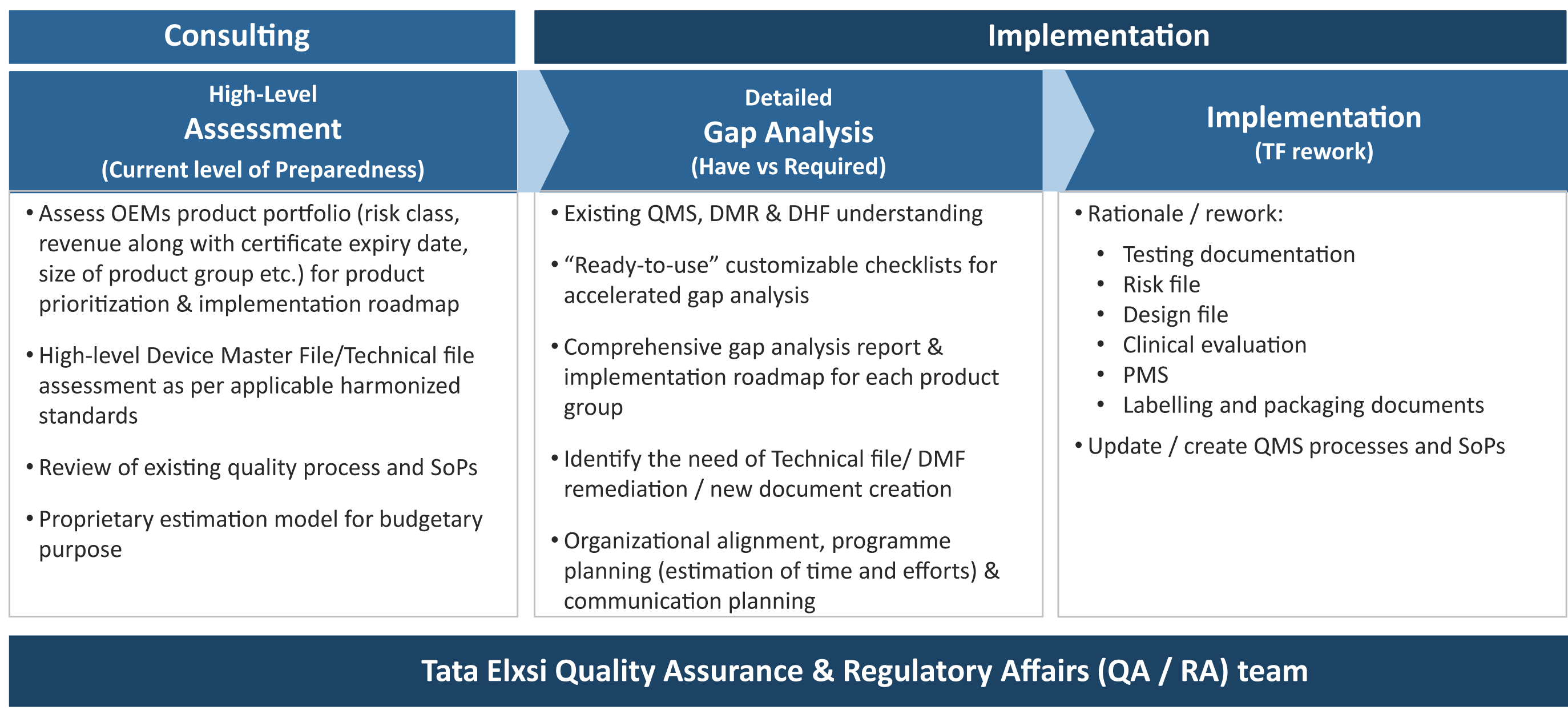
This can be used as a gap analysis tool or as an aide memoire during your transition. Gap assessments will be performed by cross functional teams depending on the size of your organization. Your bsi team is here to support you on your journey so please talk to us about your plans early on in your. Furthermore also a gap analysis of the new ivdr eu2017746 is available and we are also offer webinars and consulting.
Your first step should be to assess your current level of compliance. Greenlight guru has teamed up with eu mdr expert firm regulatory globe to offer a free mdr gap analysis tool to help companies with the transition process for compliance with new requirements for medical devices to be sold in the european market starting in 2020. Emergo can assist with this. Transitioning to the mdr might seem overwhelming and many companies dont know where to start.
Scope of new data. The mdr tool can be downloaded in english or german language. The mdr gap analysis tool supports medical device companies to implement the new medical device regulation eu2017745 in a easy way. Without an understanding of these changes and how they impact you it is easy to be.
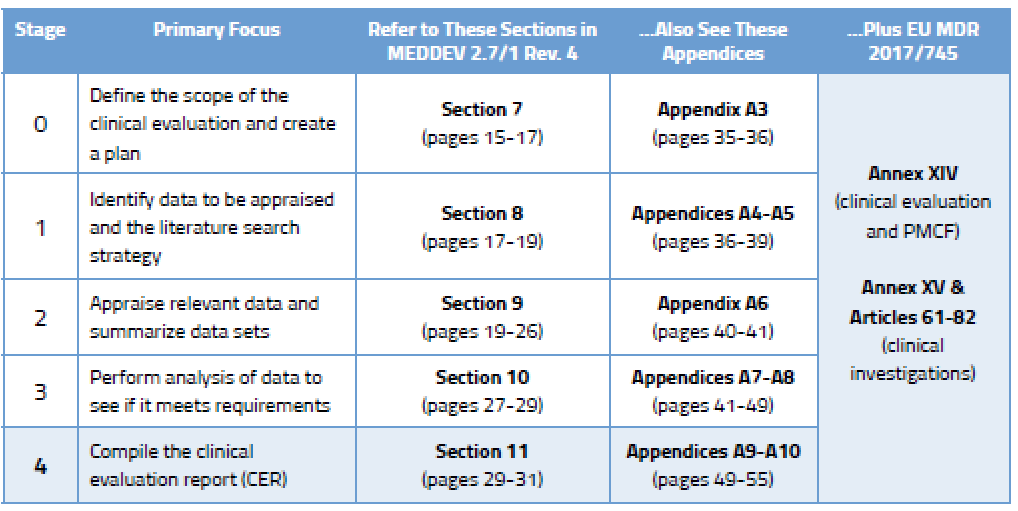
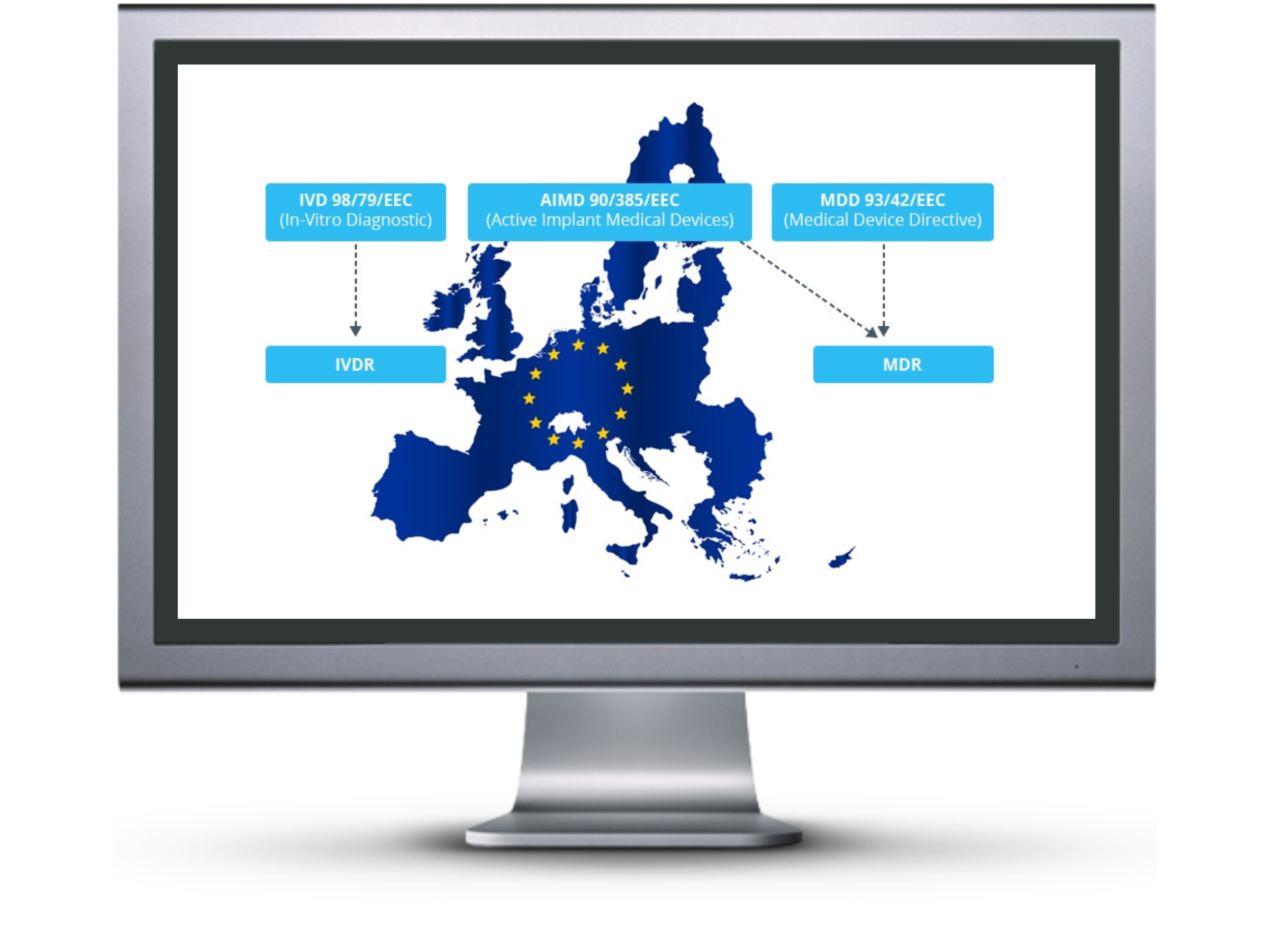
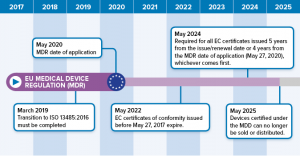
Eu mdd to mdr 2017745 transition strategy and plan. The mdr which replaces the medical devices directive. This blog provides a gap analysis tool for updating your medical device reporting procedure against the new 21 cfr 803. As mentioned in our recent blog a key first step is to conduct a thorough gap analysis to evaluate current capabilities and future requirements.
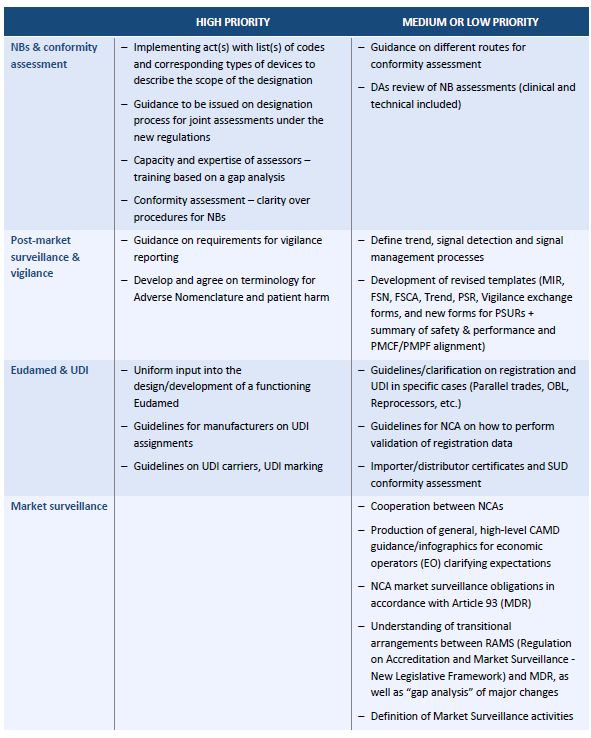
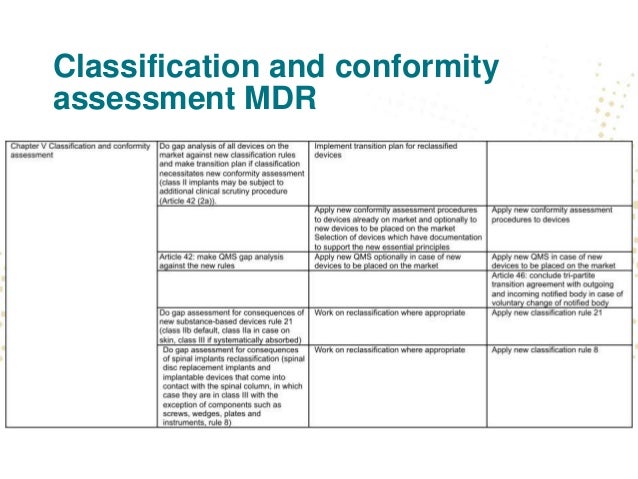
A thorough gap analysis will generate a task list for updating your procedures and documentation. A gap assessment provides a toolbox for the decision making process and should identify four key factors according to tony blank president of infinity biomedical group.