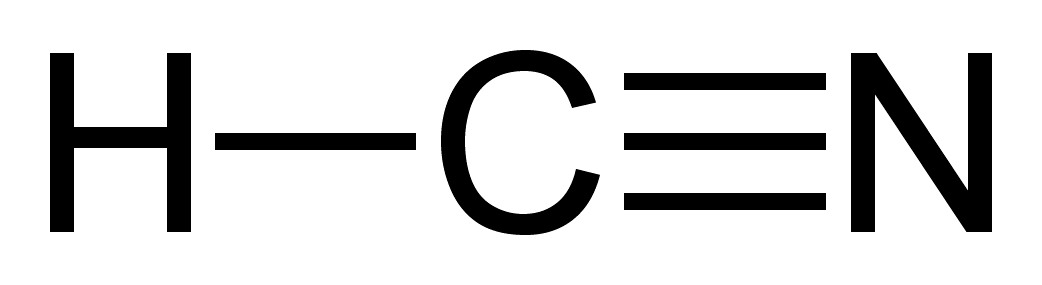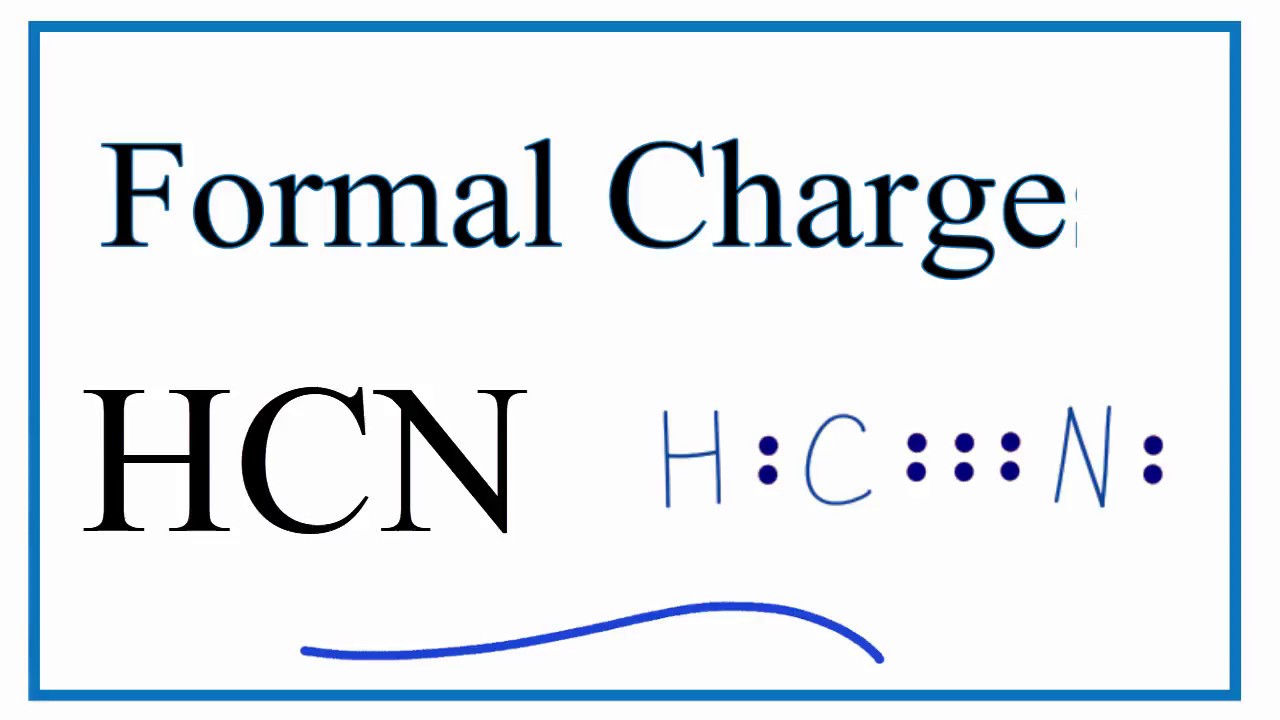Lewis Dot Diagram Hydrogen Cyanide
It can be produced by combining hydrogen chloride hcl and sodium cyanide nacn.
Lewis dot diagram hydrogen cyanide. The key is to understand the steps and practice. Lewis structures are important to learn because they help us predict. Furthermore it is a weak acid that partially ionizes in water. Hydrogen cyanide is poisonous liquid that has a faint almond like smell.
Created by makethebrainhappy this is the lewis dot structure for co2. Co32 does have a lewis dot. Once the least electronegative atom in the center fills the outer atoms move outer electron pairs to the center until they have a full octet. Often described as a pale or blue colorless liquid it has a distinctive scent that will warn those of its toxicity.
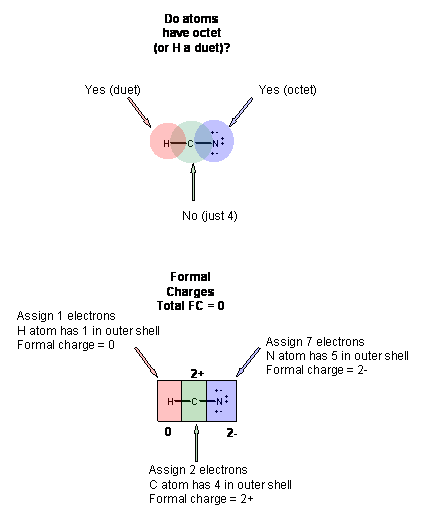
However they can make less bonds and have lone pair. Which of the answer choices shows the correct lewis dot diagram for hydrogen cyanide. It is determined by the fact that carbon makes four bonds with four valence electrons nitrogen makes five bonds with five valence electrons and hydrogen makes one bond with one valence electron. Many cyanide containing compounds are highly toxic but some are not.
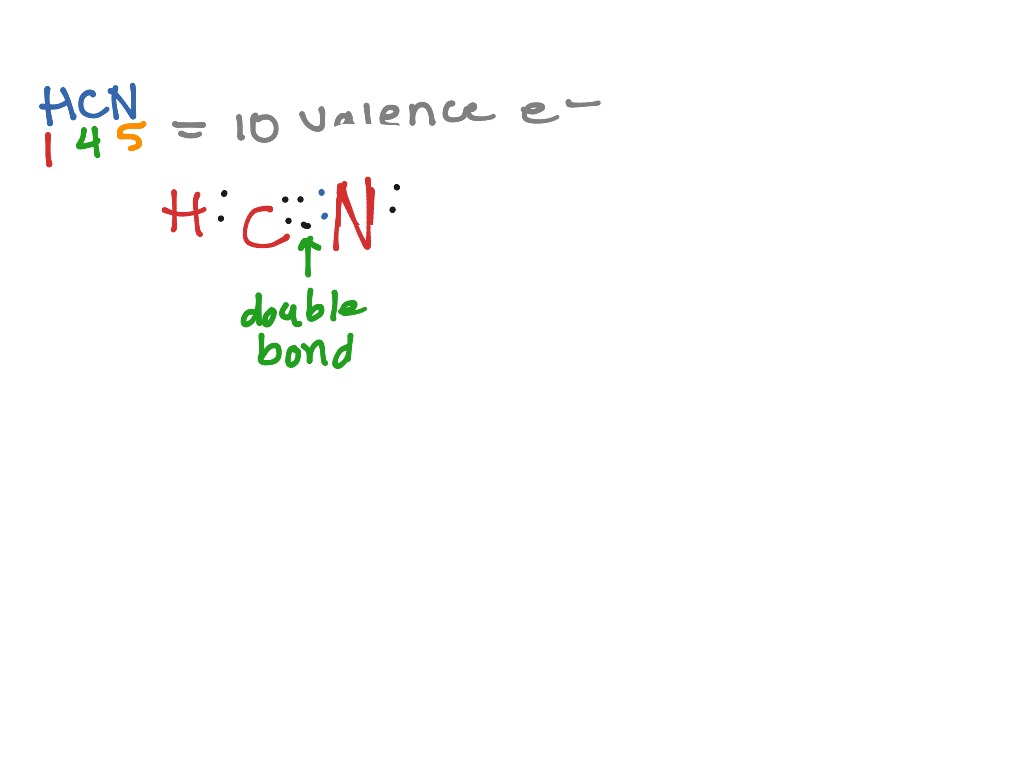
Single bondor on the computer a line between h and c. Here is an easy way and a formal way to draw the lewis structure of hcn hydrogen cyanide. What is the lewis dot structure for hydrogen cyanide. In the formal way we find how many electrons we have step 1 how many each atom needs step 2 how many of those are bonding step 3 4 and how many are lone pairs step 5.
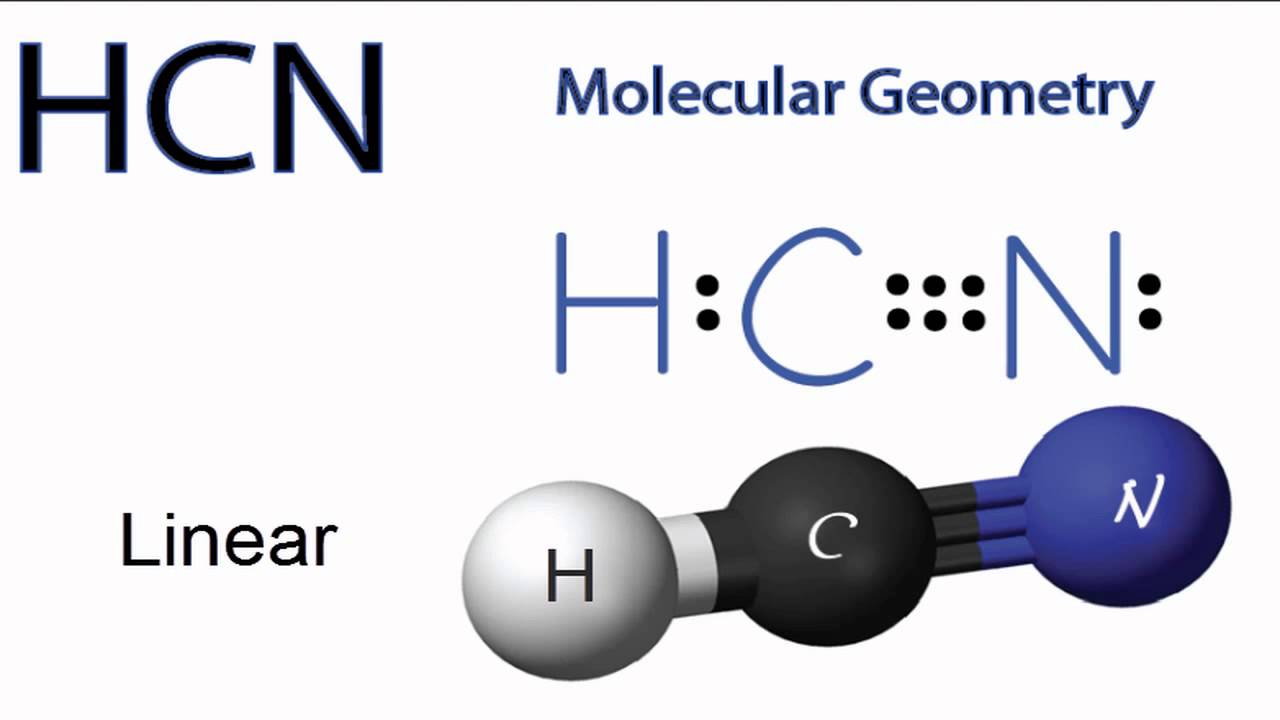
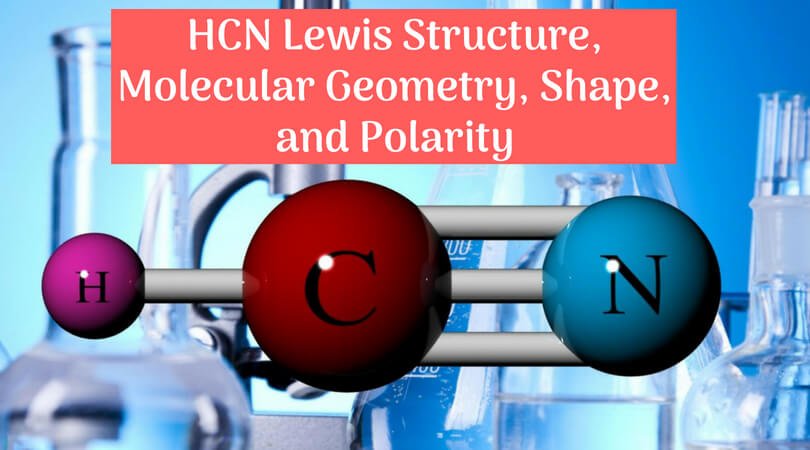
The lewis structure for hcn otherwise known as hydrogen cyanide is fairly simple. This is how lewis dot structure of hydrogen cyanide goes. Consist of a carbon atom triple bonded to a nitrogen atom. Every chemistry student has to learn how to draw lewis dot structures.
Place the carbon atom in the center and triple bond it to a nitrogen atom. The most dangerous cyanides are hydrogen cyanide hcn and salts derived from it such as potassium cyanide kcn and sodium cyanide nacn among others. It should be called like h single bond c triple bond n with a lone pair of electrons sitting on the end.