Iso 14971 Certification

Recent changes in eu medical device regulations and and activities toward a new revision of iso 14971 in 2019 have put a spotlight on risk management.
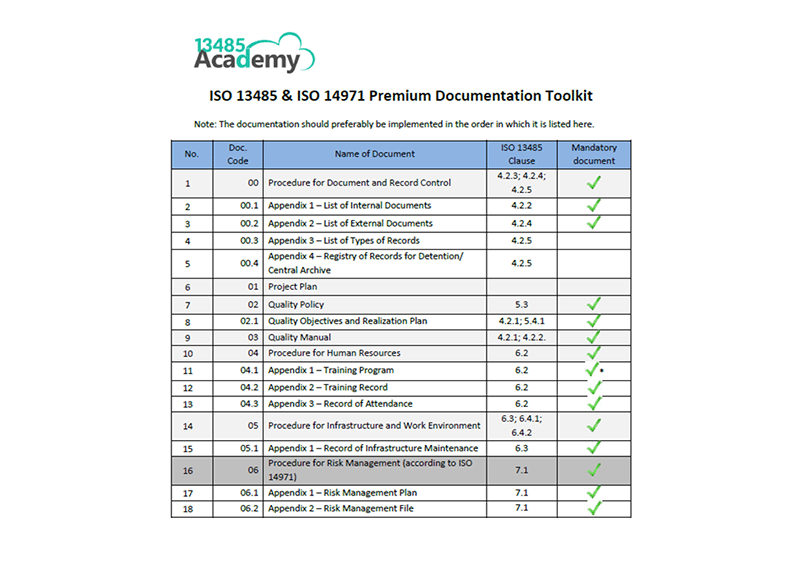
Iso 14971 certification. It defines a set of medical device risk management requirements. This standard defines the best practices throughout the entire life cycle from design to distribution and maintenance. According to clause 3 in iso 14971 top management must. Additionally iso 14971 provides a thorough explanation of terms and definitions.
In addition to applying risk management practices being familiar with other iso standards can be valuable in developing safe and effective medical devices. The process flow for risk management based on iso 14971 is shown in figure 1. The course covers all parts of the risk management process including annex z from the en iso 149712012 version of the standard as well as an orientation on the iso 14971 risk managements relation to tools and techniques such as fmea p fmea and fta. Iso 14971 certification can help you develop and maintain a risk management system and process in the healthcare industry.
Medical devices including in vitro diagnostic medical devices. Iso 13485 defines risk based on iso 14971 as the combination of the probability of occurrence of harm and the severity of that harm risk management process through iso 14971. Companies with this certification communicate a commitment to quality to both customers and regulators. Iso 14971 medical device risk management training.
Iso 14971 certification to maximize the effectiveness of your risk management system its important to ensure that your processes meet the requirements for iso 14971 compliance. There is more emphasis on total life cycle management than ever before. Iso 14971 addresses risk management and is the international standard designed for the medical device industry.



.jpg)


.jpg)