Iso 14385 Certification

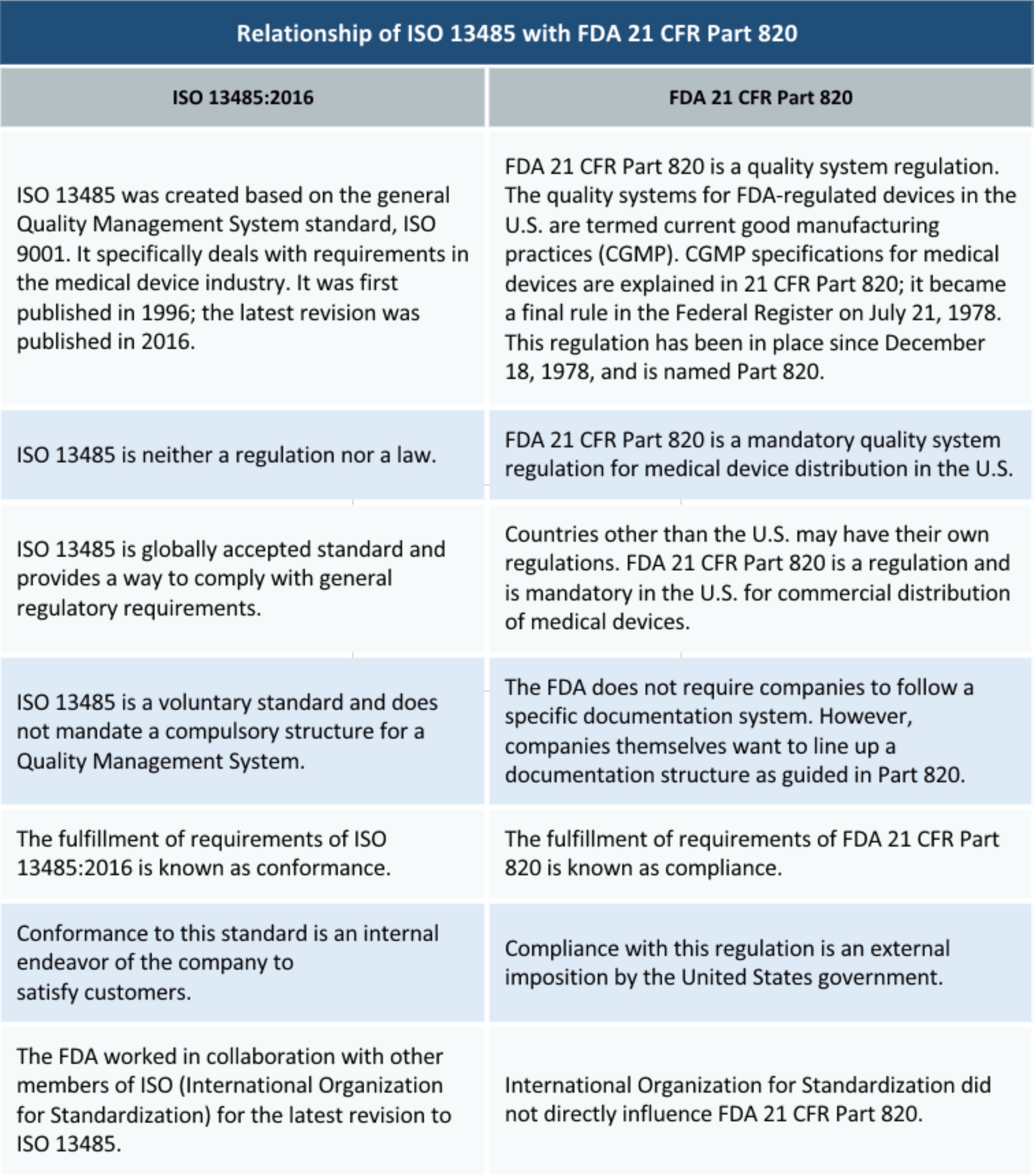
Iso 134852003 specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide medical devices and related services that consistently meet customer requirements and regulatory requirements applicable to medical devices and related services.
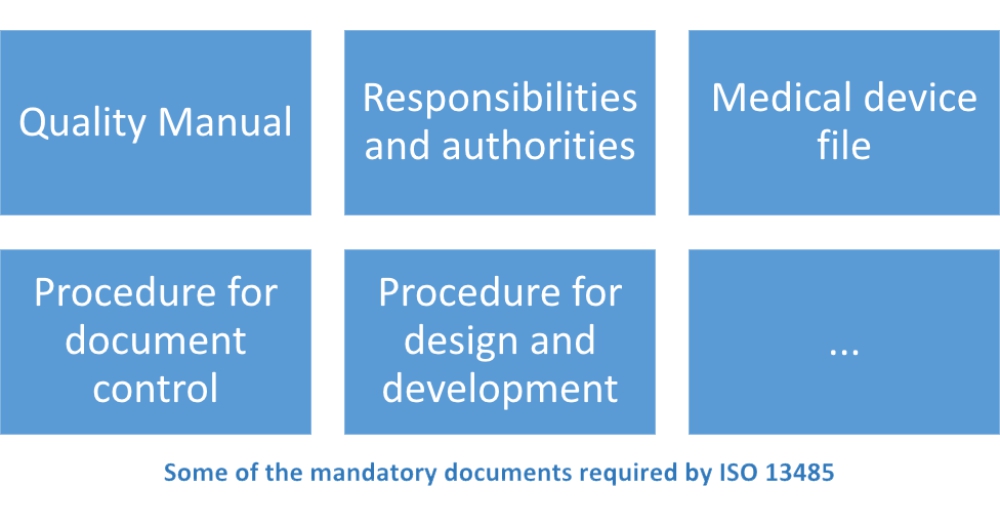
Iso 14385 certification. Iso 13485 medical devices quality management systems requirements for regulatory purposes is an international organization for standardization iso standard published for the first time in 1996. It can also be used by internal and external parties such as certification bodies to help them with their auditing processes. Certification to the standard requires an organizations quality management system to pass a third party medical device single audit program or mdsap audit. The iso 13485 standard is an effective solution to meet the comprehensive requirements for a qms.
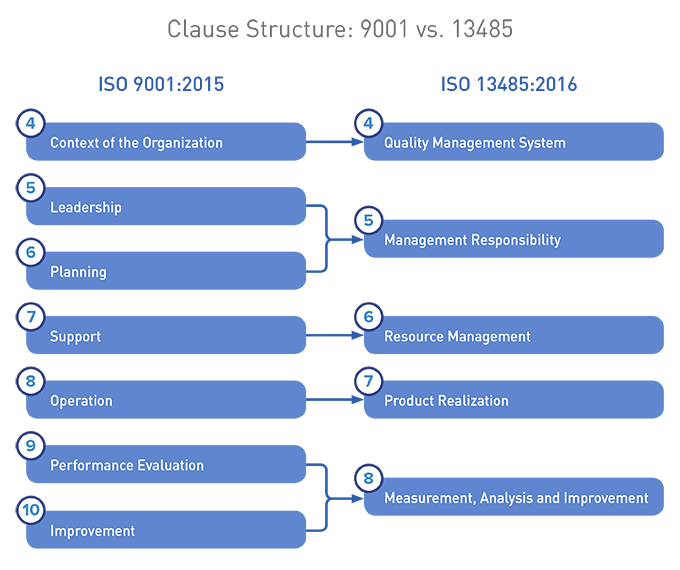
It represents the requirements for a comprehensive quality management system for the design and manufacture of medical devices. Iso 13485 is the best internationally accepted model a medical device organization can implement to help demonstrate compliance to laws and regulations of the medical device industry. Who is iso 13485 for. Iso 13485 contains requirements that are essential for any organization operating at any tier in the medical device and pharmaceutical supply chain.
Iso 13485 training courses classes webinars online training powerpoints materials all in one place. Iso 134852016 is the standard for a quality management system qms for the design and manufacture of medical devices. If youve ever struggled to find iso 13485 training courses webinars online webinars and training materials like powerpoint presentations this is a great site for you. Iso 13485 certification is especially relevant to manufacturers that wish to demonstrate applicable regulatory requirements and by organizations whose services support medical device manufacturers.
One of the medical device industrys most widely used international standards for quality management iso 13485 is evolving with the publication of a new version of the standard by the international organization for standardization iso. Iso 13485 is the quality management system standard accepted as the basis for ce marking medical devices under european directives and regulations. Iso 13485 is designed to be used by organizations involved in the design production installation and servicing of medical devices and related services.