Fda Software Validation Templates

A look at the top five most common software validation and documentation questions asked by others in fda regulated industries and best practices for meeting the guidelines.
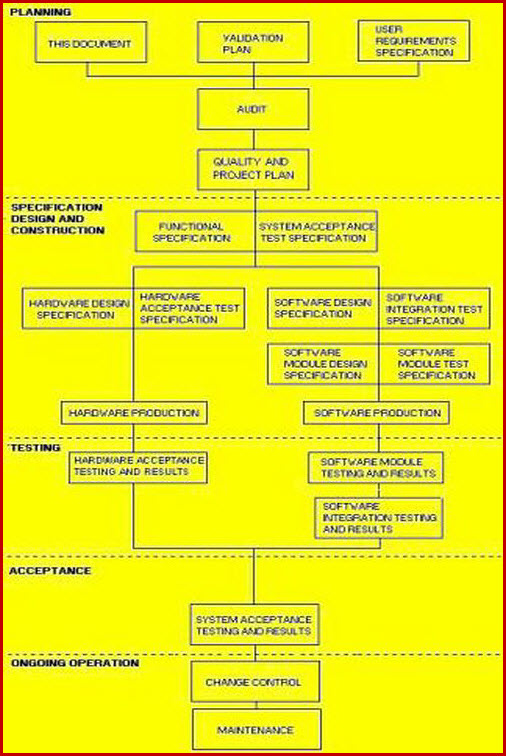
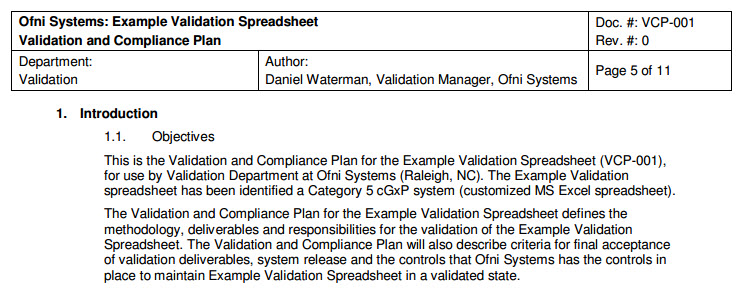
Fda software validation templates. Validation strategy the validation strategy and thus the extent of the validation activities depends ultimately on the maturity and complexity of the computer software components implied in ispe gamp5 and partly fda 21 cfr 21168b 6 1. If your regulatory personnel have experience in leading an fda validation project that included the back end erp software then you can leverage your internal resources by having them take the lead in the process. Many times software vendors will try to sell prepackaged validation documentation. Why cant i just purchase validation documentation.
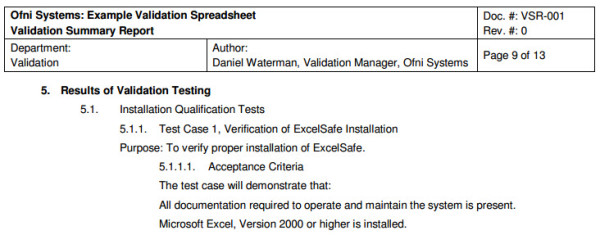
Our fda validation process templates provide guidance to streamline the workload of your internal fda validation personnel. For the fda document general principles of software validation final guidance for industry and fda staff download ms word format 877 kb 118 pages also available in pdf format item no. Rcg010awsep published march 2002 description evidence product checklist for the fda document general principles of software validation final guidance for industry and fda staff. What to do to validate quality computer systems.
This guidance outlines general validation principles that the food and drug administration fda considers to be applicable to the validation of medical device software or the validation of. 1 41202 conversion to word 2000 format. These sops and templates also incorporate industry standards and best practices such as those found in pics and gamp. Computer software as part of the computer system dictates the hardware on which to be executed.
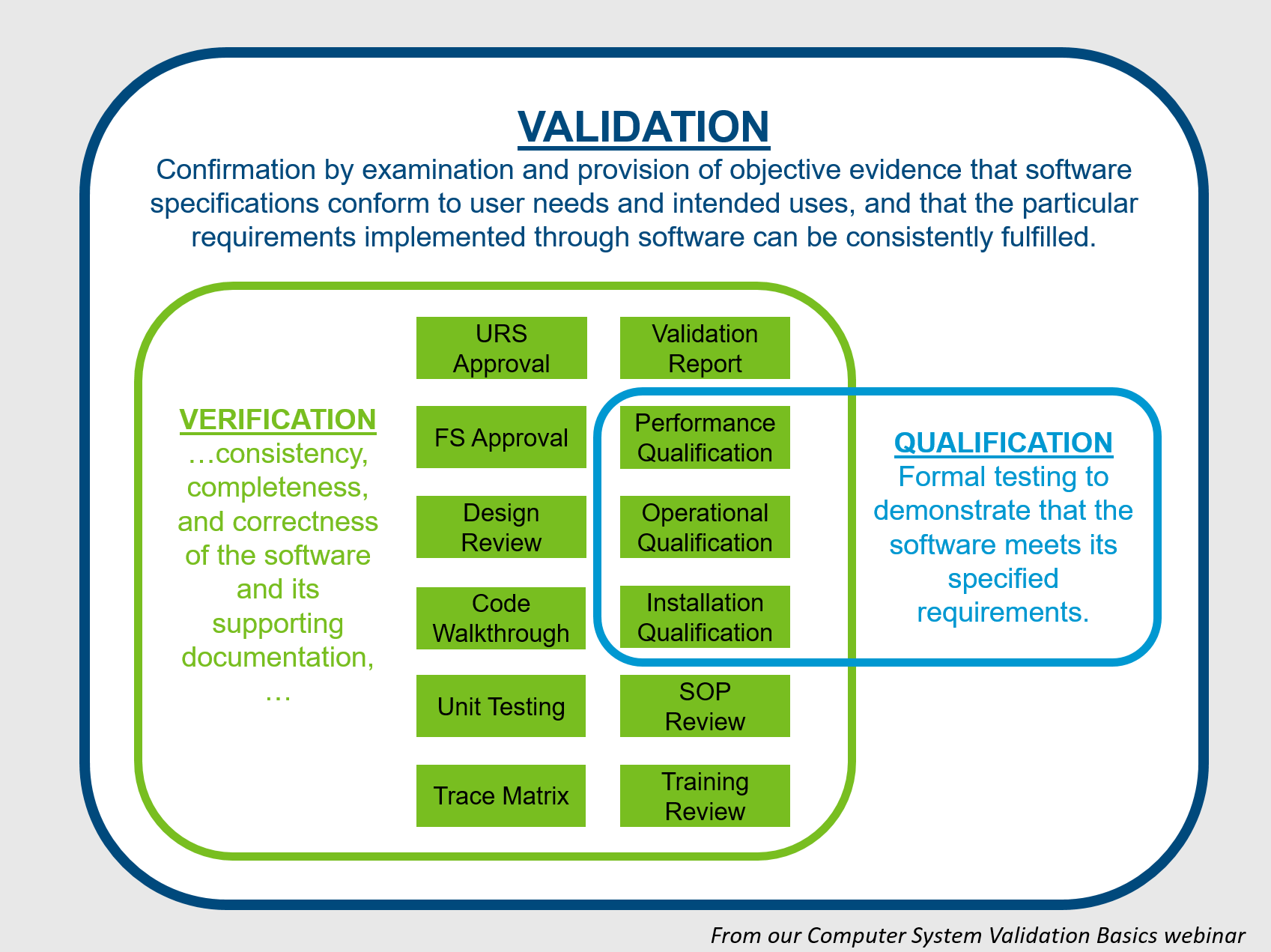
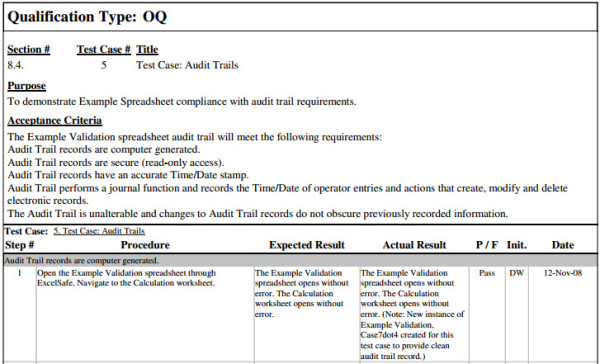
Page 2 guidance for industry and fda staff general principles of software validation in that case the party with regulatory responsibility ie the device manufacturer needs to assess the. Validation verification and testing. The validation center library offers computer system validation sops and templates to expedite your implementation of a software validation program that complies with the expectations of the fda ema and ich.