Enzyme Worksheet Answer Key What Is A Catalyst

Print activation energy and catalysts worksheet 1.
Enzyme worksheet answer key what is a catalyst. This worksheet and quiz will give you an opportunity to test your. Enzyme worksheet 1 what is a catalyst. What is a catalyst. Try all keys with all locks and answer the following questions about set 1 of locks.
The enzyme is consumed by the reaction 1. The energy needed to start a chemical reaction is called activation energy. Activation energy is the energy needed to start a reaction. Speeds up a chemical reaction by reducing the amount of.
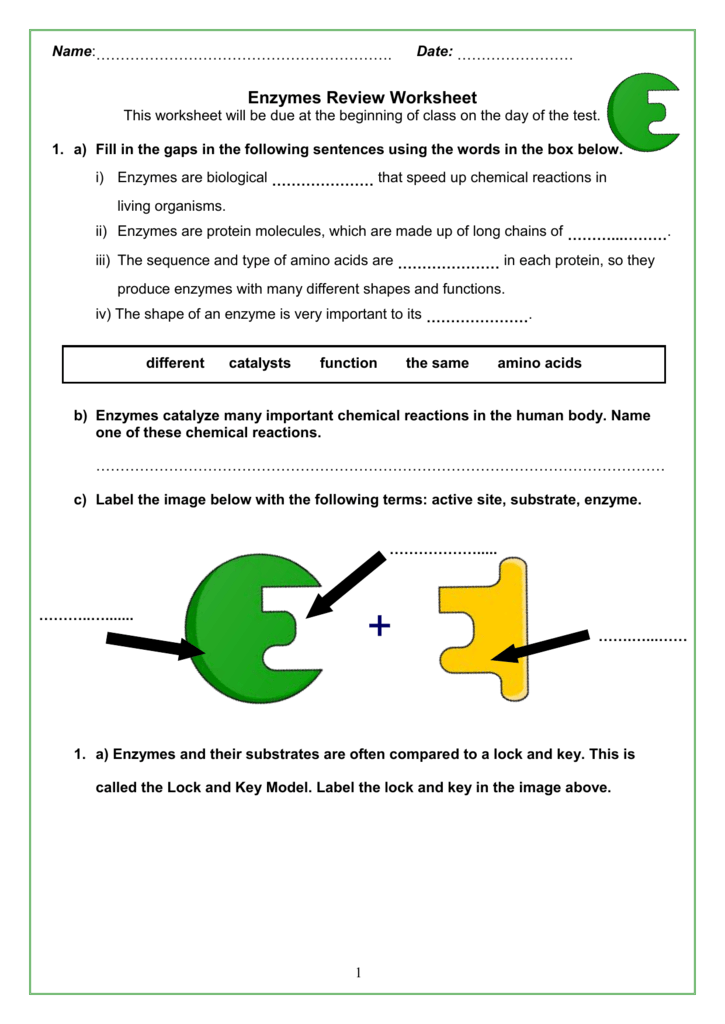
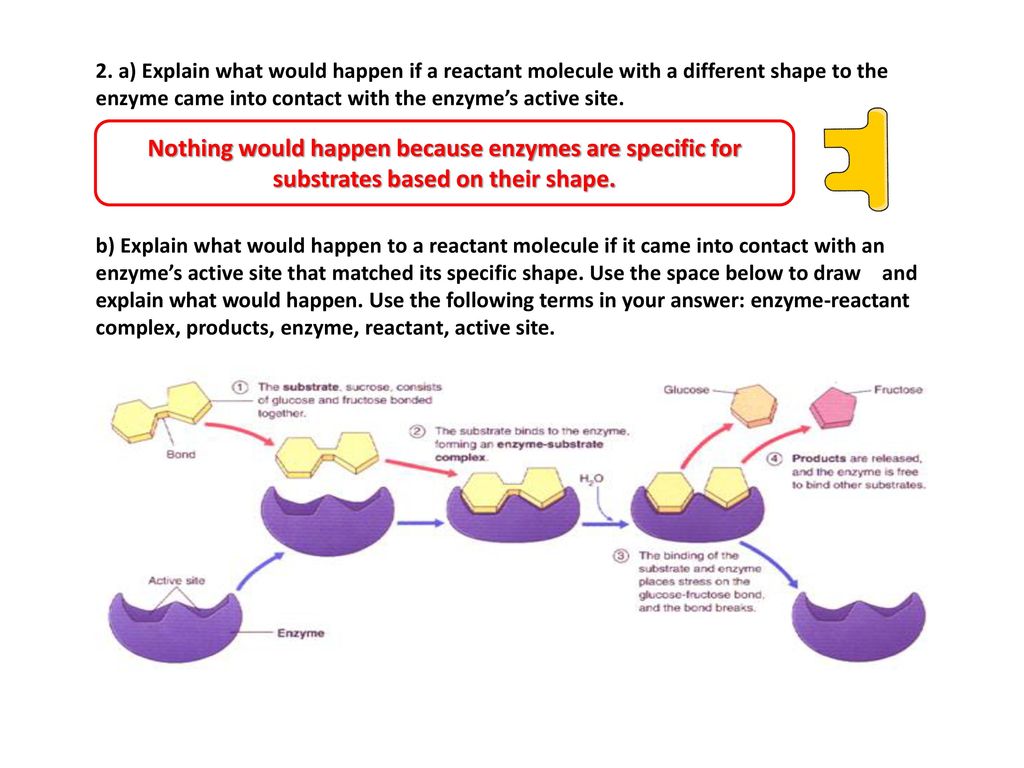
The objective of this activity is to introduce the concept of enzymes and their functions through a lock and key model by using real locks and keys as an analogy. Model of enzyme activity that explains how a particular enzyme will only fit with one particular type of substrate. After reading the information on enzymes answer the following questions. Which of the following does not apply to an enzyme.
2 what is an enzymeenzymes act as catalysts and they speed up chemical reactions. What does a catalyst do. Procedure part 11. Why do enzymes generally bind to only one.
What are the main molecules that act as catalysts in living things called. Substrates bind in the active site 2. The shape of the enzyme remains unchanged 4. Answers enzymes questions 1.
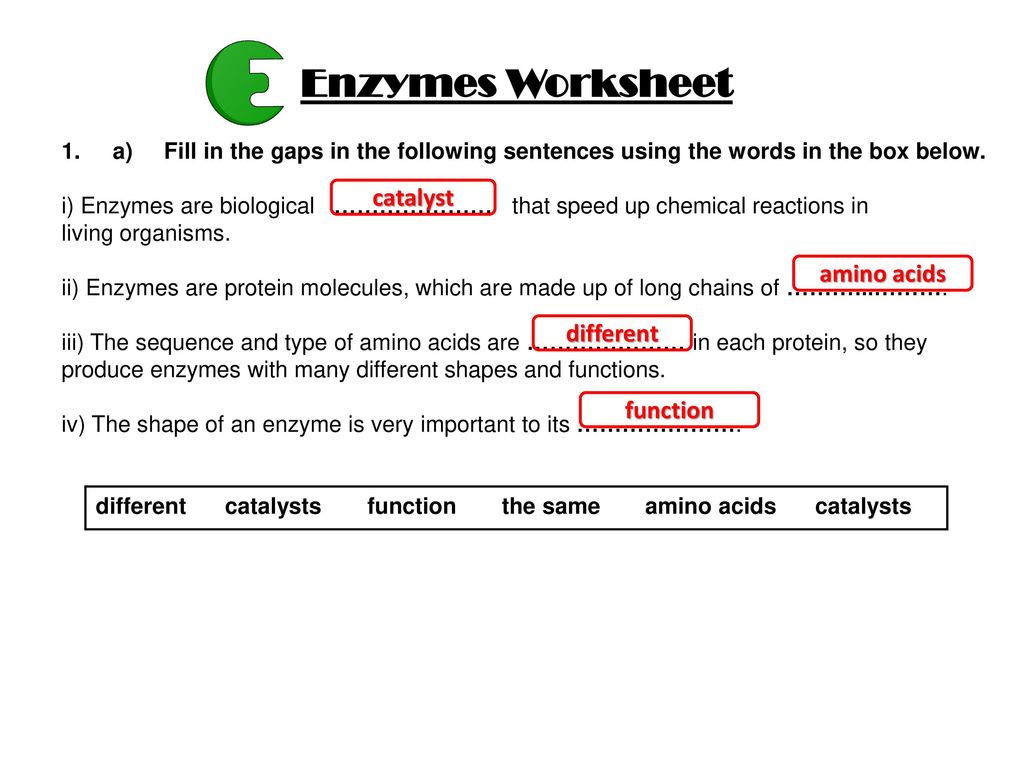
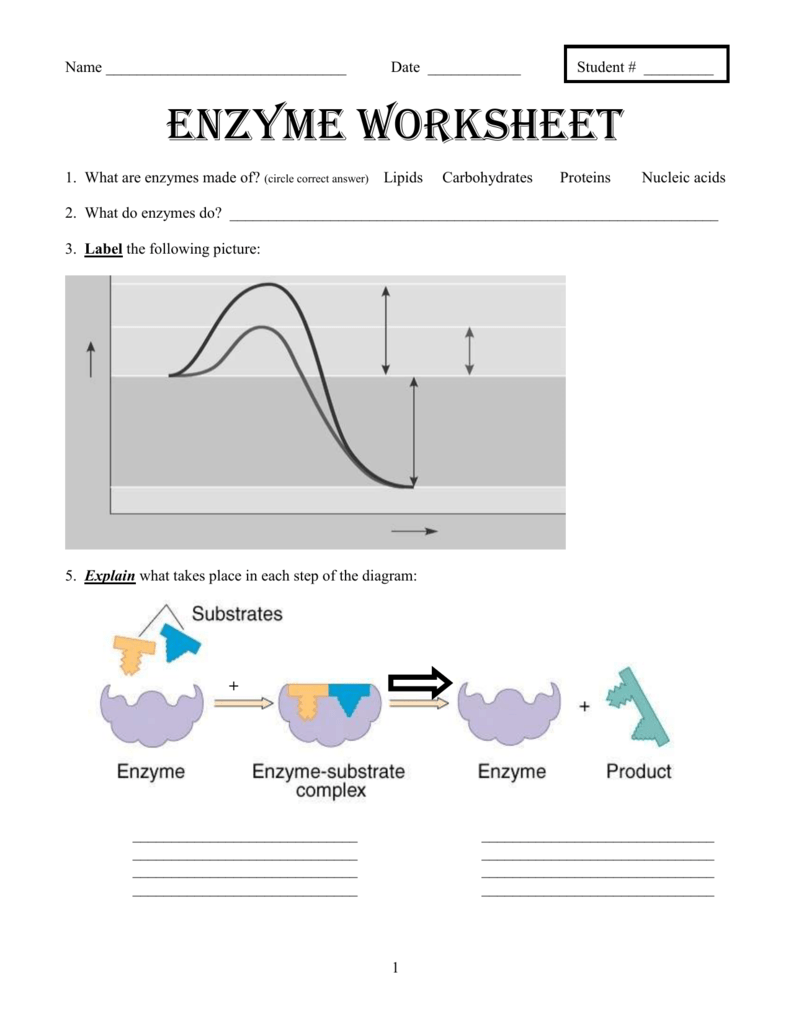
Enzymes are made of what kind of macromolecule. Enzymes a catalyst is a substance that speeds up a chemical reaction by reducing the amount of activation energy needed to start that reactionenzymes are the biological molecules proteins or rna that act as catalysts in a living organism. Catalysts speed up the chemical reactions by lowering the activation energy without causing any permanent change to the reaction. A catalyst is a compound that speeds up a chemical reactions 3.
Set 1 of locks and keys will be provided by your teacher. When an enzyme catalyzes a reaction. What is an enzyme. An enzyme is a protein that acts as a catalyst to speed up the rate of a chemical reaction by lowering the activation energy.
If we know that manganese dioxide is a catalyst in the decomposition of hydrogen peroxide then how does manganese dioxide affect this reaction. All of the above apply to an enzyme 1. A catalyst is a substance that speeds up the rate of a chemical reaction. Products bind in the active site 3.
The seemingly simple act of breaking down food molecules to release energy is actually a series of.