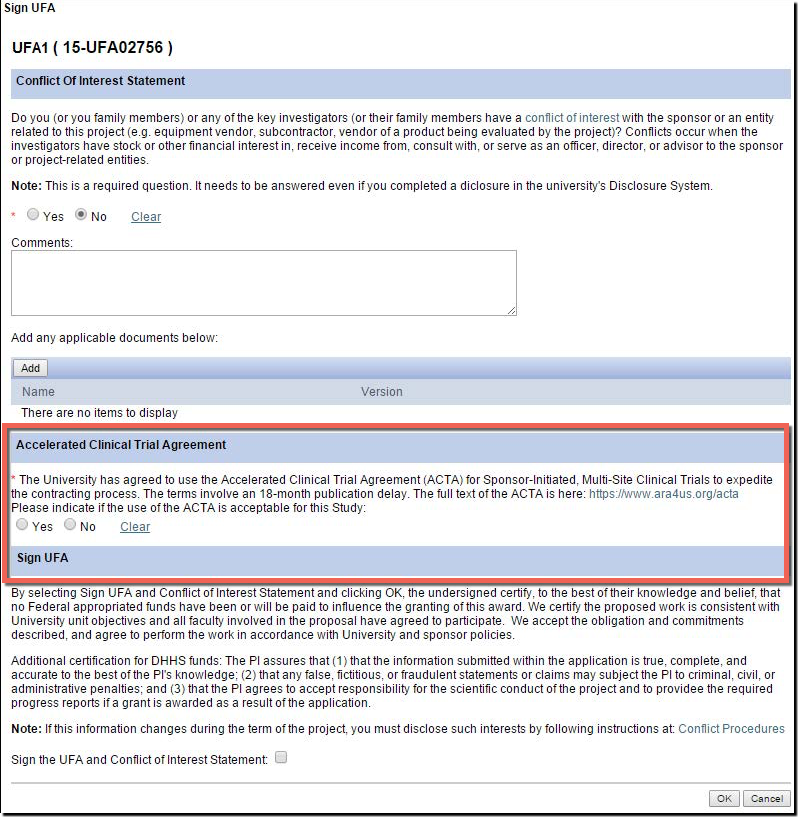
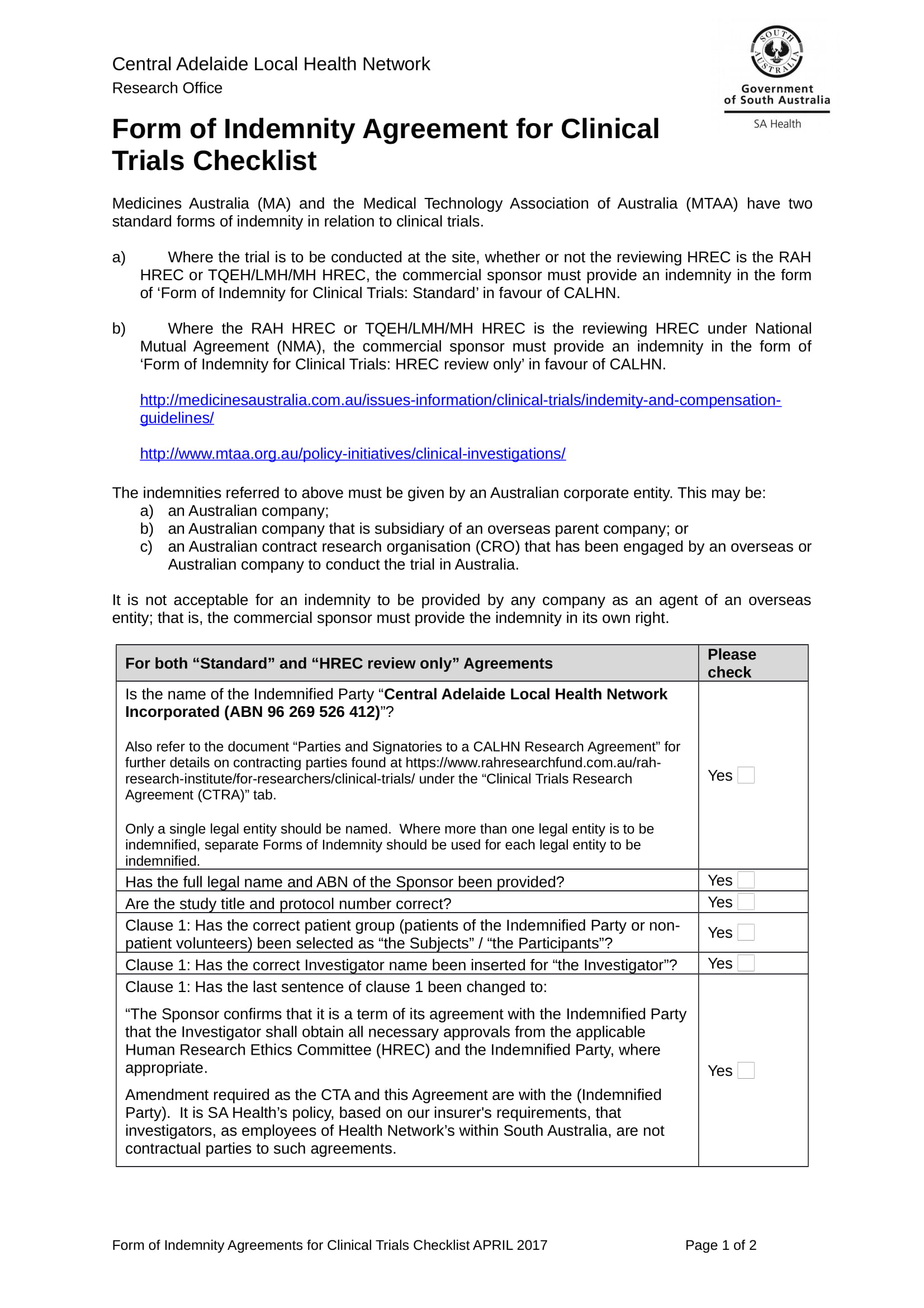
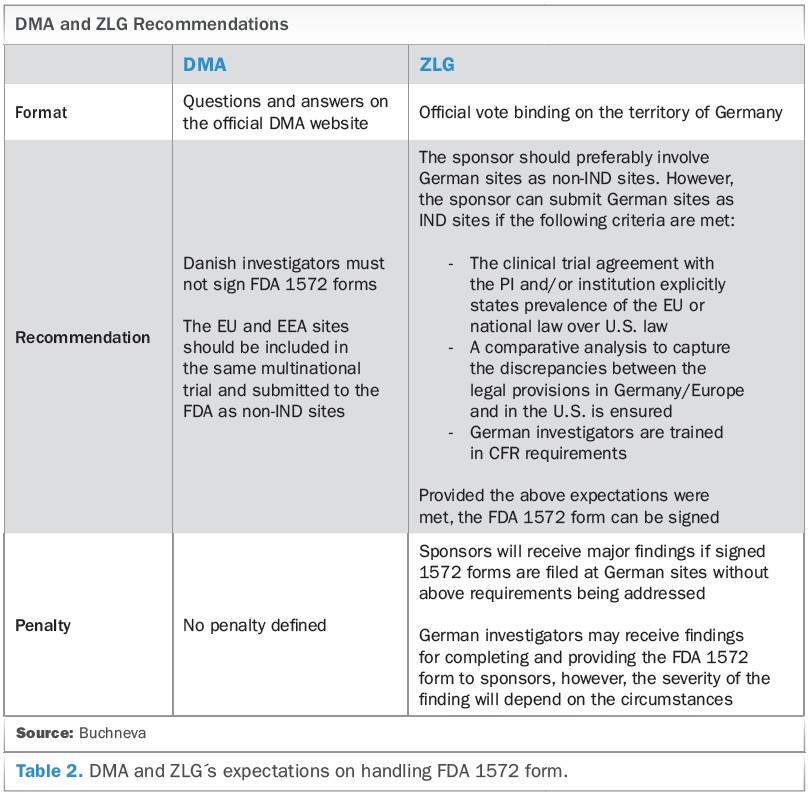
Clinical Trial Agreement Template

A clinical trial agreement cta is a legally binding agreement that manages the relationship between the sponsor that may be providing the study drug or device the financial support and or proprietary information and the institution that may be providing data andor results publication input into further intellectual property.
Clinical trial agreement template. When specific commercial products like medications or cosmetics need to be tested on people a clinical trial might be necessary. It is important to protect the interests of both partiesdownload pdf. This section of the clinical trial agreement template provides you with an area to document all ownership rights between parties as well as any other rights to property involved in the clinical trial agreement. The pennsylvania state university and.
Hershey medical center located at 500 university drive hershey pa 17033 located at. A clinical trial agreement is a legally binding agreement between the company that makes the product known as the sponsor and an appropriate institution such as a research university. The february 2018 revised model clinical trial agreement mcta and clinical research organisation model clinical trial agreement cro mcta templates are designed to be used without modification for industry sponsored trials in nhshsc patients in hospitals throughout the uk health service. The purpose of this agreement is to lay down the terms of the clinical trial.
A clinical trial agreement is an agreement between an institution and a sponsor of a clinical trial as to how the clinical trial shall be conducted.