Capa Form Template

Guidelines for writing a capa version june 2018 capa template oria how to create a corrective and preventive action plan capa a.
Capa form template. Corrective and preventative actions template. Corrective and preventive action plan capa report form for medical device for fda compliance with 21 cfr part 820100 slideshare uses cookies to improve functionality and performance and to provide you with relevant advertising. Download capa template capa format. Template version 304 october 2010.
This blog reviews 15 tips for creating an effective capa form including capa source description of issue investigation of root cause and more. Free download of capa template how to write a capa how to write a capa report preventive action what is a capa form. Smith t created date. Title and acronym if available sponsor.
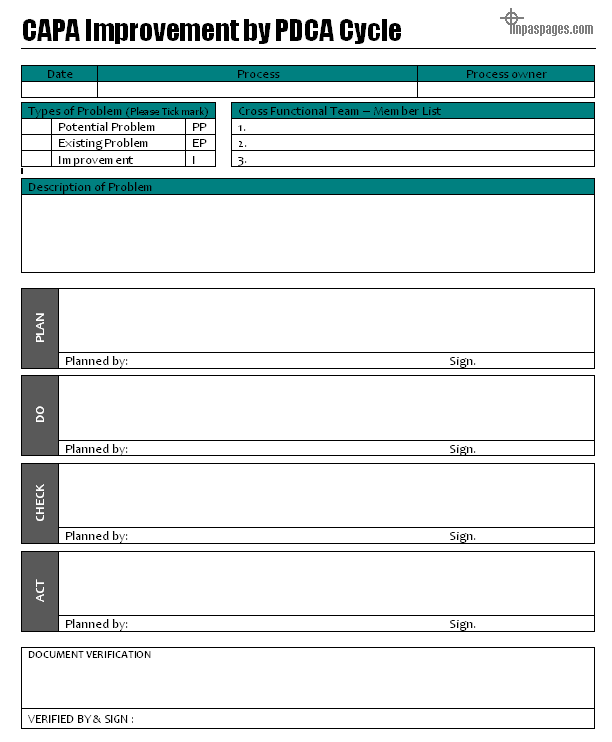
Free download qms ehs templateformat. Capa may be applied to a variety of aspects of product development such as design production product testing and post market use. This can be used by compliance officers when formulating a corrective action to resolve the issue and discussing preventive actions to lower the risk of its recurrence. Corrective and preventive action format capa with example.
Smith t last modified by. Anyone in your company could be assigned to a capa but not everyone is a capa expert. The reason for creating a great capa form is to improve the effectiveness of your capa process. Download corrective action format.
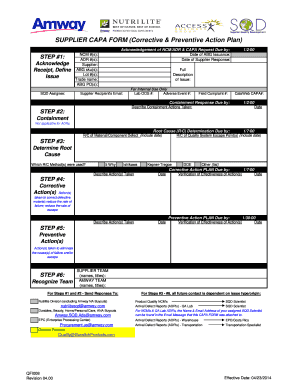
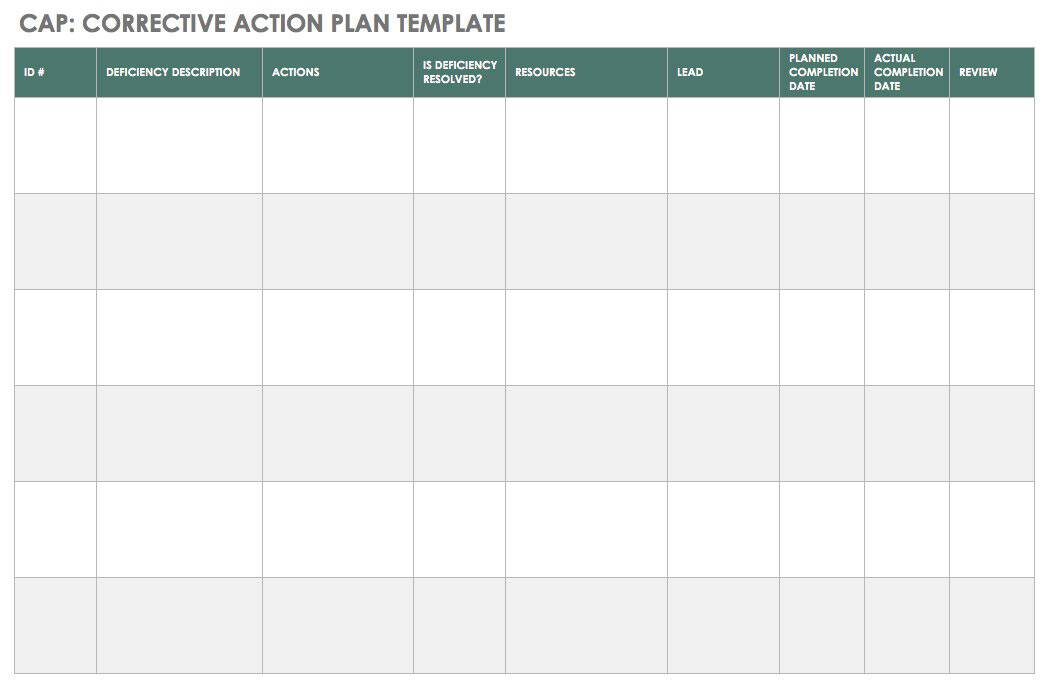
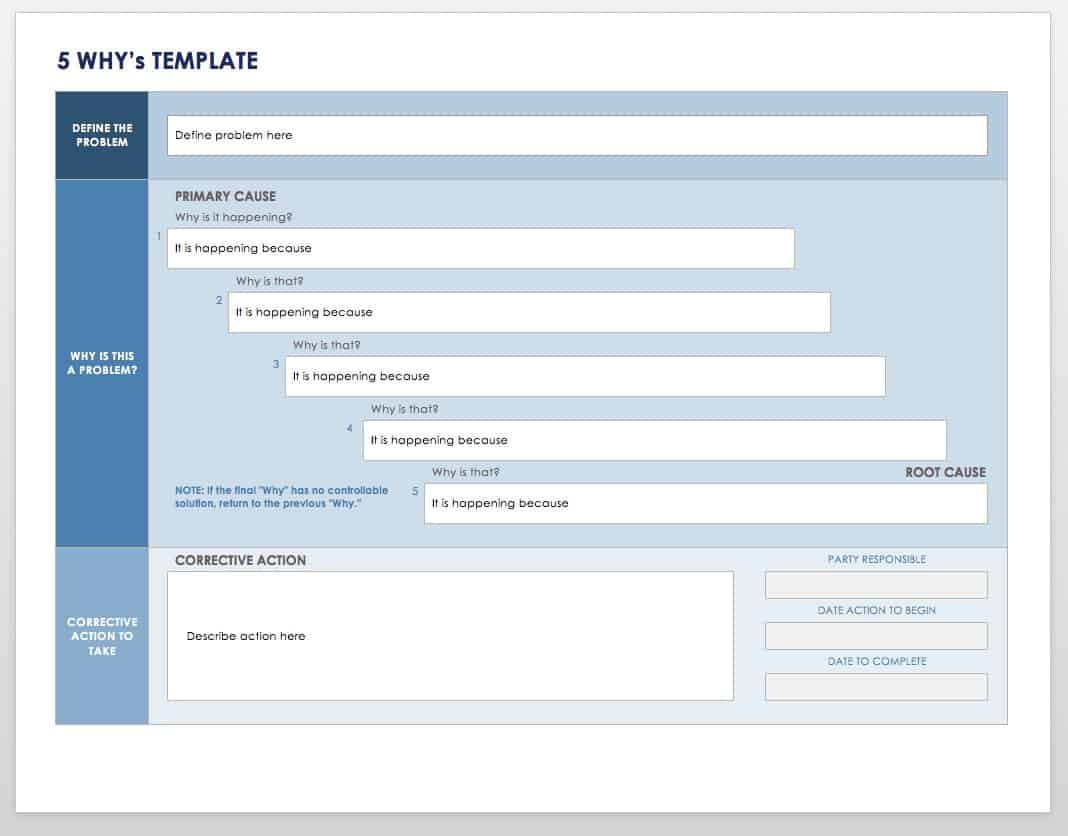
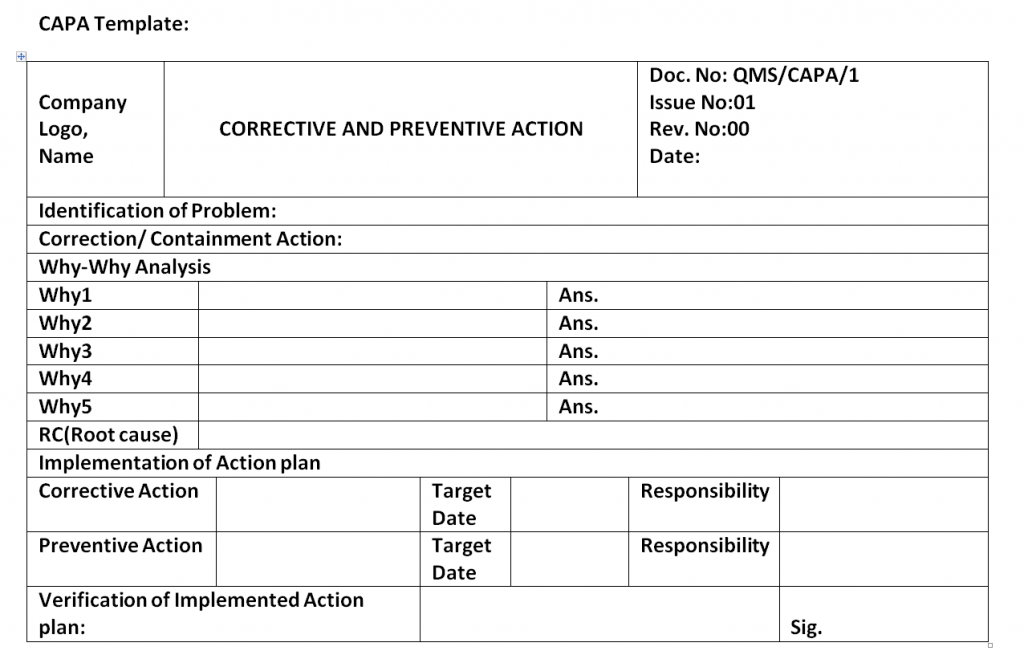
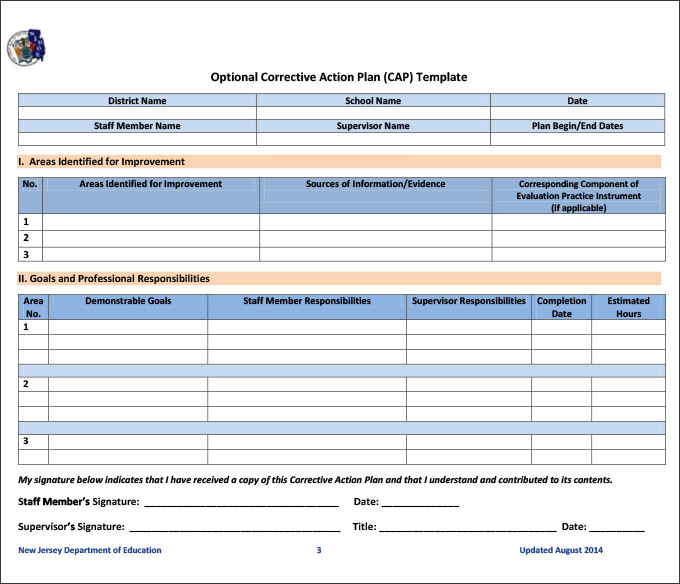
Is written to identify a discrepancy or problem in the conduct of the clinical research study note the root cause of the identified problem identify the corrective. Every great capa plan needs an equivalent capa reporting form to organize and document the quality management strategy and outcome from beginning to end. A capa report form is designed to help identify address and prevent the occurence of regulatory and organizational non conformance. Capa report template.
The capa concept is also integral to the current good manufacturing process cgmp an approach advocated by the fda. Corrective action and preventive action capa plan template. Capa may also be applied in product packaging distribution and shipping.